
A manometer is a device used to measure pressure in a fluid, particularly a double-legged liquid column gauge used to compare the pressures of two fluids.Vaporization is the process through which a liquid phase changes to a gas phase.A liquid’s boiling point is the temperature at which its vapour pressure falls equivalent to the pressure exerted on the liquid by its surroundings (air).


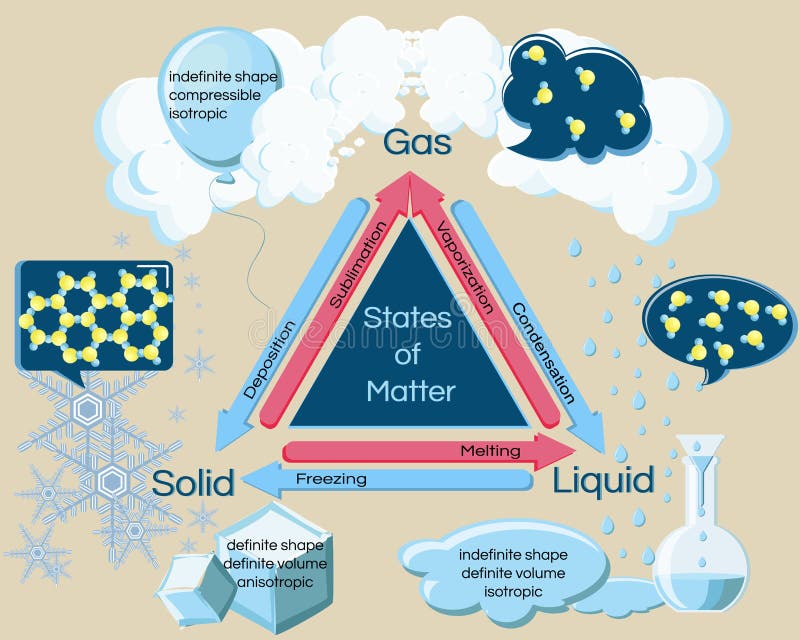
Similarly, as a material changes from a gas phase to a liquid phase, its density levels must shift from lower to higher, requiring the substance to release or lose energy to bring the molecules closer together. If a solid changes to a liquid, it must absorb energy to push the molecules into a larger, more fluid volume. In terms of mechanism, latent heat is the effort required to overcome the attractive forces that hold molecules and atoms together in a substance. Latent heat is associated with a heat property called enthalpy.Īn important point to consider regarding latent heat is that the substance’s temperature remains constant. It could be from a gas to a liquid or liquid to solid and vice versa. Latent heat is the heat or energy absorbed or released during the phase change process. It's difficult to find an example of this since it requires a sub-zero temperature. When the reaction takes place, it changes into a soil state without being changed into a liquid, which is the intermediate state. In this case, the substance is in a gaseous state. Depositionĭeposition is the opposite of sublimation. For example, the naphthalene balls used in the households are an example of sublimation. Sublimation is the process by which matter goes from solid to gas without first being transformed into liquid.


 0 kommentar(er)
0 kommentar(er)
